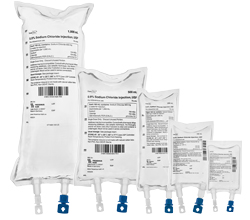
Unit of Sale NDC
65219- | Unit of Use NDC
65219- | Product Number | Description | Strength
(Concentration) | Fill Volume
(Container Size) | Pack Size | Min. Order Qty. | Product Label | Package Insert |
| 472-20 | 472-05 | 416620 | Single Dose freeflex® bag | 0.9% (4,500 mg per 500 mL)
(9 mg per mL) | 500 mL
(500 mL) | 20 | 1 x 20 | | |
| 474-10 | 474-05 | 416610 | Single Dose freeflex® bag | 0.9% (9,000 mg per 1,000 mL)
(9 mg per mL) | 1,000 mL
(1,000 mL) | 10 | 1 x 10 | | |
| 470-30 | 470-05 | 416630 | Single Dose freeflex® bag | 0.9% (2,250 mg per 250 mL)
(9 mg per mL) | 250 mL
(250 mL) | 30 | 1 x 30 | | |
| 468-50 | 468-05 | 416650 | Single Dose freeflex® bag | 0.9% (900 mg per 100 mL)
(9 mg per mL) | 100 mL
(100 mL) | 50 | 1 x 50 | | |
| 466-60 | 466-05 | 416660 | Single Dose freeflex® bag | 0.9% (450 mg per 50 mL)
(9 mg per mL) | 50 mL
(100 mL) | 60 | 1 x 60 | | |
freeflex® is a registered trademark of Fresenius Kabi USA, LLC